It’s not the amount of fluoride
It’s the length of time it is available
For fluoride to be beneficial for longer than the time of brushing, fluoride needs to be deposited and slowly released.
Professor Ten Cate
The following is an extract from an interview with Professor Hill from Queen Mary University of London. The full article is available here
The problem with fluoride toothpaste
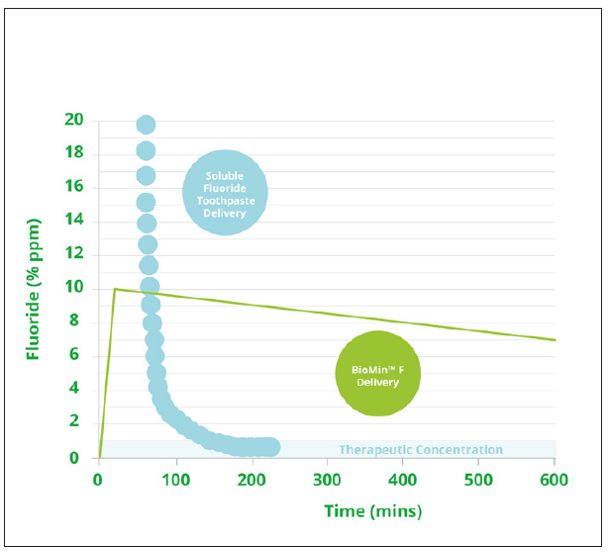
Professor Hill’s experiments have demonstrated that when conventional fluoride toothpaste containing a soluble fluoride such as sodium fluoride or sodium monofluorophosphate is used, there is an immediate ‘high’ of fluoride in the mouth, but that this drops rapidly as the toothpaste is washed away by salivary flow, so that after around only 100 minutes the amount of fluoride that remains is below therapeutic levels. Even at high concentrations, the fluoride is rapidly washed away, so the effect is only short term. Fluoride varnish, too, is only effective for a few days. A further drawback is that high concentrations of fluoride form calcium fluoride (also known as fluorite) instead of fluorapatite, which is what is required for effective remineralisation. In large quantities fluorite can form a whitish crust on the tooth surface, which was previously thought to act as a reservoir of fluoride, but Professor Hill’s research has shown that this is not the case. ‘It is completely insoluble, and does not release fluoride at all,’ he explains.
By contrast, the fluoride contained within the glass structure of Biomin F is released slowly over around 12 hours and is therefore used more effectively. ‘As it dissolves, the glass structure in Biomin F provides a slow release vehicle for the fluoride, calcium and phosphate together, enabling it to form fluorapatite, which is more stable and resistant to acid conditions,’ he says.
How fluoride works in Biomin F
Biomin F has been developed to address three key problems in dental health: hypersensitivity, caries and dental erosion, caused by loss of tooth enamel or demineralisation. Under normal conditions, the hydroxyapatite mineral in tooth enamel is in dynamic equilibrium with the calcium, phosphate and hydroxyl ions in saliva, but under acidic conditions, such as following an acidic drink, this equilibrium is shifted, the pH in the mouth falls and demineralisation can occur.
As the bioactive glass in Biomin F gradually dissolves, releasing phosphate, calcium and fluoride ions, these work in concert with the saliva to restore the equilibrium. Even more clever, at a lower pH the glass dissolves faster, so that the effect kicks in more rapidly. Professor Hill summarises: ‘This smart response means that if the user consumes an acidic drink, Biomin F dissolves faster to protect the teeth against acid dissolution.’
Rigorous testing
Professor Hill’s labs at Queen Mary University of London use state-of-the-art equipment for analysis and have tested and timed the action of Biomin F both in buffer solution, containing no calcium or phosphate ions, and in artificial saliva (AS). The effects were impressive. In buffer, the glass converts to fluorapatite in around six hours, but in artificial saliva this starts occurring in under an hour after brushing, as shown by X-ray
diffraction.
Queen Mary has a dedicated probe for its NMR spectrometer, allowing the team to measure fluorine and study how it converts to fluorapatite – important because of its increased stability and acid resistance. Here too it was possible to see that the fluoride is converted to fluorapatite in around six hours in buffer and under 45 minutes in AS. Biomin F continues to remineralise tooth enamel for approximately 12 hours but some effects are still continuing at 24 hours after brushing. In order for the glass to dissolve slowly where it’s needed, the toothpaste has to stay on the teeth. The polymer used in Biomin F increases the viscosity of the toothpaste, but also chemically bonds to both the calcium in the tooth enamel
and the calcium in the Biomin F, so that it sticks to the tooth surface and remains in place to release the fluoride, calcium and phosphate ions for several hours.
Sensitivity and internal tubule occlusion
As the glass particle size is very small, these particles are able to enter the dentinal tubules and work to occlude these. Fluorapatite forms preferentially on the apatite rich walls of the peritubular dentine within the tubules gradually occluding them, an effect still visible after acid challenge. As the fluorapatite occludes the dentinal tubules, it reduces the flow of fluid through them, known as hydraulic conductance, which is the cause of sensitivity. Studies in the Queen Mary labs have shown that the fluorapatite formed by the dissolution of the glass in Biomin F is more resistant to acid challenge than hydroxycarbonated apatite formed from soluble fluoride in conventional toothpastes, and so the tubules remain occluded more completely. The hydraulic conductance shows a greater percentage reduction as well as faster remineralisation rates than other toothpastes tested, says Professor Hill.
Professor Hill and his research team’s work in developing Biomin F has shown clearly that it is not quantity of fluoride that improves its efficacy, but quality – the way that it is delivered. Incorporating fluoride within the structure of the bioactive glass, combining it with phosphate and calcium ions to enable quicker production of stable, acid-resistant fluorapatite, and adhering the product to the teeth so that it can dissolve slowly where it can deposit fluorapatite most effectively, is the key to its effectiveness. Biomin F is a smart toothpaste, using new technology to deliver efficient remineralisation at levels of fluoride far lower than conventional toothpastes.

Scanning electron micrograph showing BioMin F tubule occlusion before & after acid challenge.
You have read all about BioMin F, give BioMin F a try and see why its ProductReview.com.au’s No.1 rated Dental Care Product. Consumers please visit or shop. Oral health care professionals and pharmacies please send us an email and we can organise a wholesale account to be set up for your practice / pharmacy.
